Modelling of 2,4-dichlorophenol, an emerging pollutant removal from water by adsorption onto sugarcane bagasse biochar using response surface methodology
Abstract
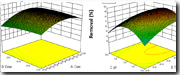
This study evaluated the potential of sugarcane bagasse biochar as a low-cost adsorbent for removing 2, 4-dichlorophenol (2, 4-DCP), an emerging pollutant from water using adsorption modelling and response surface methodology. It aimed to determine optimal combinations of concentration, contact time, pH and dose for maximizing contaminant uptake in batch and column systems. Batch adsorption experiments were planned using a Box–Behnken response surface experimental design with initial 2, 4-DCP concentration (25, 50, and 75 mg L–¹), contact time (20, 40, and 60 min), solution pH (5, 7, and 9), and biochar dosage (6.25, 12.5, and 25 mg). Fixed-bed column studies were conducted under continuous flow to assess dynamic performance and breakthrough behaviour. Adsorption equilibrium, kinetics and column behaviour were analysed using standard isotherm and kinetic models, supported by surface and functional-group characterization. The optimized batch conditions produced removal efficiencies of about 95% with high monolayer adsorption capacity on a homogeneous biochar surface. The equilibrium data followed a monolayer adsorption model, while kinetic analysis indicated rapid uptake controlled primarily by surface-site availability. Column studies showed high dynamic capacity and well-defined breakthrough characteristics under the tested flow conditions. Sugarcane bagasse biochar proved to be an efficient and technically suitable material for removing phenolic contaminants such as 2, 4-DCP from water. The findings demonstrate a productive use of agro-industrial waste for water purification and support its application in practical treatment units for removing emerging pollutants in aquatic environments.
References
Ahmed MB, Zhou JL, Ngo HH, Johir MAH, Sun L, ... Belhaj D (2018) Sorption of hydrophobic organic contaminants on functionalized biochar: protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. Journal of Hazardous Materials 360: 270–278.
Amin FR, Huang Y, He Y, Zhang R, Liu G, Chen C (2016) Biochar applications and modern techniques for characterization. Clean Technologies and Environmental Policy 18(5): 1457–1473.
Box GE, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2(4): 455–475.
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82(11): 1518–1532.
Celcey Z (2019) The embryotoxicity of some phenol derivatives on zebrafish, Danio rerio. Caspian Journal of Environmental Sciences 17(1): 11–22.
Chatterjee N, Sinha D, Lemma-Dechassa M, Tan S, Shogren-Knaak MA, Bartholomew B (2011) Histone H3 tail acetylation modulates ATP-dependent remodeling through multiple mechanisms. Nucleic Acids Research 39(19): 8378–8391.
Chen H, Yang X, Wang H, Sarkar B, Shaheen SM, ... Rinklebe J (2020) Animal carcass-and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: a trial for reclamation and improvement of degraded soils. Journal of Environmental Management 261: 110246.
Chiron S, Minero C, Vione D (2007) Occurrence of 2, 4-dichlorophenol and of 2, 4-dichloro-6-nitrophenol in the Rhône river delta (Southern France). Environmental Science & Technology 41(9): 3127–3133.
Czech B, Kończak M, Rakowska M, Oleszczuk P (2021) Engineered biochars from organic wastes for the adsorption of diclofenac, naproxen and triclosan from water systems. Journal of Cleaner Production 288: 125686.
Dong W, Xing J, Chen Q, Huang Y, Wu M, ... Xing B (2024) Hydrogen bonds between the oxygen-containing functional groups of biochar and organic contaminants significantly enhance sorption affinity. Chemical Engineering Journal 499: 156654.
Ensley HE, Barber JT, Polito MA, Oliver AI (1994) Toxicity and metabolism of 2, 4‐dichlorophenol by the aquatic angiosperm Lemna gibba. Environmental Toxicology and Chemistry 13(2): 325–331.
Freundlich HMFZ (1906) Stoechiometrie und Verwandtschaftslehre. Zeitschriftfuer Physikalische Chemie 57: 385–470 (in German).
Ghosh SK (2016) Biomass & bio-waste supply chain sustainability for bio-energy and bio-fuel production. Procedia Environmental Sciences 31: 31–39.
Gopinath KP, Vo DVN, Gnana Prakash D, Adithya Joseph A, Viswanathan S, Arun J (2021) Environmental applications of carbon-based materials: a review. Environmental Chemistry Letters 19(1): 557–582.
Hall RA, Hara M, Knoll W (1996) Isomerization and acid− base behavior in polyion complex langmuir− blodgett films. Langmuir 12(10): 2551–2555.
Ho YS, Ng JCY, McKay G (2000) Kinetics of pollutant sorption by biosorbents. Separation and Purification Methods 29(2): 189–232.
Hu Y, Li D, Ma X, Liu R, Qi Y, ... Huang D (2021) Effects of 2, 4-dichlorophenol exposure on zebrafish: implications for the sex hormone synthesis. Aquatic Toxicology 236: 105868.
Imade O, Ilesanmi BV, Ogunwole GO, Elekofehinti OO, Souza MCO, ... Adeyemi JA (2024) Effects of 2, 4-dichlorophenol on non–specific immunity, histopathological lesions, and redox balance in African catfish, Clarias gariepinus (Burchell, 1822). Journal of Toxicology and Environmental Health, Part A 87(11): 480–495.
Kumar G, Kumar S, Paul T, Pal P, Shukla SP, ... Pradeep S (2024a) Ecotoxicological risk assessment of triclosan, an emerging pollutant in a riverine and estuarine ecosystems: a comparative study. Marine Pollution Bulletin 205: 116667.
Kumar K, Sarkar P, Paul T, Shukla SP, Kumar S (2024b) Ecotoxicological effects of triclosan on Lemna minor: bioconcentration, growth inhibition and oxidative stress. Environmental Science and Pollution Research 31(45): 56550–56564.
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society 40(9): 1361–1403.
Latch DE, Packer JL, Stender BL, VanOverbeke J, Arnold WA, McNeill K (2005) Aqueous photochemistry of triclosan: formation of 2, 4‐dichlorophenol, 2, 8‐dichlorodibenzo‐p‐dioxin, and oligomerization products. Environmental Toxicology and Chemistry 24(3): 517–525.
Li Q, Yu J, Chen W, Ma X, Li G, ... Deng J (2018) Degradation of triclosan by chlorine dioxide: reaction mechanism, 2, 4-dichlorophenol accumulation and toxicity evaluation. Chemosphere 207: 449–456.
Li Y, Li Q, Wu C, Luo X, Yu X, Chen M (2020) The inappropriate application of the regression Langmuir Qm for adsorption capacity comparison. Science of the Total Environment 699: 134222.
Liu S, Ma Y, Liu H, Wang S (2025) The adsorption–desorption behavior of 2, 4-dichlorophenol on microplastics in waters with different salinity. International Journal of Environmental Science and Technology 22(4): 2565–2576.
Matolia J, Shukla SP, Kumar S, Kumar K, Singh AR (2019) Physical entrapment of chitosan in fixed-down-flow column bed enhances triclosan removal from water. Water Science and Technology 80(7): 1374–1383.
Mohammadi A, Sandberg M, Venkatesh G, Eskandari S, Dalgaard T, ... Granström K (2019) Environmental analysis of producing biochar and energy recovery from pulp and paper mill biosludge. Journal of Industrial Ecology 23(5): 1039–1051.
Moussavi G, Khosravi R (2011) The removal of cationic dyes from aqueous solutions by adsorption onto pistachio hull waste. Chemical Engineering Research and Design 89(10): 2182–2189.
Pan X, Wei J, Zou M, Chen J, Qu R, Wang Z (2021) Products distribution and contribution of (de) chlorination, hydroxylation and coupling reactions to 2, 4-dichlorophenol removal in seven oxidation systems. Water Research 194: 116916.
Petroutsos D, Katapodis P, Samiotaki M, Panayotou G, Kekos D (2008) Detoxification of 2, 4-dichlorophenol by the marine microalga Tetraselmis marina. Phytochemistry 69(3): 707–714.
Ponnuchamy M, Kapoor A, Jacob MM, Awasthi A, Mukhopadhyay M, ... Prabhakar S (2025) Adsorptive removal of endocrine disruptor bisphenol A from aqueous environment using sugarcane bagasse derived biochar. Journal of the Taiwan Institute of Chemical Engineers 166: 105216.
Prasannamedha G, Kumar PS, Shivaani S, Kokila M (2022) Sodium alginate/magnetic hydrogel microspheres from sugarcane bagasse for removal of sulfamethoxazole from sewage water: batch and column modeling. Environmental Pollution 307: 119523.
Rishika MS, Kumar S, Paul T, Shukla SP, Bharti VS, Kumar K (2024) Performance evaluation of sugarcane bagasse biochar based fixed bed column for removal of triclosan from aqueous solution in presence of humic acid: mechanism, kinetics and safety. Journal of Water Process Engineering 64: 105672.
Sharma P, Sharma S, Sharma SK, Jain A, Shrivastava K (2024) Review on recent advancement of adsorption potential of sugarcane bagasse biochar in wastewater treatment. Chemical Engineering Research and Design 206: 428–439.
Song B, Gong J, Tang W, Zeng G, Chen M, ... Yang Y (2020) Influence of multi-walled carbon nanotubes on the microbial biomass, enzyme activity, and bacterial community structure in 2, 4-dichlorophenol-contaminated sediment. Science of the Total Environment 713: 136645.
Sun K, Kang F, Waigi MG, Gao Y, Huang Q (2017) Laccase-mediated transformation of triclosan in aqueous solution with metal cations and humic acid. Environmental Pollution 220: 105–111.
Tahir H, Sultan M, Akhtar N, Hameed U, Abid T (2016) Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. Journal of Saudi Chemical Society 20: S115–S121.
Tan XF, Zhu SS, Wang RP, Chen YD, Show PL... Ho SH (2021) Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chinese Chemical Letters 32(10): 2939–2946.
Thomas HC (1944) Heterogeneous ion exchange in a flowing system. Journal of the American Chemical Society 66(10): 1664–1666.
Tran HN, Bollinger JC, Lima EC, Juang RS (2023) How to avoid mistakes in treating adsorption isotherm data (liquid and solid phases): Some comments about correctly using Radke-Prausnitz nonlinear model and Langmuir equilibrium constant. Journal of Environmental Management, 325, p.116475
Wahyudi DP, Ghaisani SV, Bismo S (2020) Degradation of 2, 4-dichlorophenol in aqueous solution by ozonation in the presence of iron oxide compound in bubble column reactor. Engineering Journal 24(4): 183–193.
Weber Jr WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division 89(2): 31–59.
Wu P, Ata-Ul-Karim ST, Singh BP, Wang H, Wu T, ... Chen W (2019) A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 1(1): 23–43.
Xia X, Hua C, Xue S, Shi B, Gui G, ... Guo L (2016) Response of selenium-dependent glutathione peroxidase in the freshwater bivalve Anodonta woodiana exposed to 2, 4-dichlorophenol, 2, 4, 6-trichlorophenol and pentachlorophenol. Fish & Shellfish Immunology 55: 499–509.
Yoon YH, Nelson JH (1984) Application of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. American Industrial Hygiene Association Journal 45(8): 509–516.
Zafeer MK, Menezes RA, Venkatachalam H, Bhat KS (2024) Sugarcane bagasse-based biochar and its potential applications: a review. Emergent Materials 7(1): 133–161.
Copyright (c) 2026 The Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.





